| The interaction between nuclear radiation and matter is called a "nuclear reaction" (although gamma rays can also interact with the electrons of an atom through photoatomic reactions). Nuclear reactions are described by specifying the type of the incident radiation, the nuclear target, the products of the reaction, the probability that the reaction will take place, which is called the "cross section," and the distributions in energy and angle of the reaction products. |
Nuclear Radiation Types
There are several different kinds of "radiation," including
nuclear particles, that can cause nuclear reactions:
|
What is an eV, KeV, or MeV?
|
Throughout the nuclear data field, you will see the energies of nuclear
particles expressed in "electron volts," or eV. Quite often, you will
see KeV (1000 eV), MeV (one million eV), or even GeV (109 eV).
An electron volt is the energy acquired by a charged particle with unit
charge (e.g., an electron or a proton) when it falls through a
potential difference of one volt. It is equal to 1.60207x10-12
erg. Some interesting energies expressed in this way include thermal neutrons at room temperature (average energy of .0253 eV), neutrons produced by fission (around 2 MeV), neutrons produced by the d-t fusion reaction (14 MeV), and protons used in medical radiotherapy (up to 200 MeV). |
Targets for Nuclear Reactions
|
The most interesting targets for nuclear radiations (including particles)
are atomic nuclei, although charged particles do react with atomic
electrons (slowing down, stopping power). Nuclei are usually named by
giving the name of the element they belong to, or the number of protons
that they contain, together with the total number of protons and
neutrons in the nucleus. Thus, you might see 27Al,
Al-27, 208Pb, or 20882. Sometimes a nuclide
as either target or reaction product may exist in a compartively
long-lived excited state called an "isomer." Isomers are often denoted
by adding the suffix "m", e.g., Pm-148m. In many experiments
and practical nuclear devices, the target might be an element, which
contains some combination of the various stable isotopes. As an
example, Cu-nat, or natCu, contains 69% 63Cu and
31% 65Cu. For more information on nuclei, isotopes, and isomers, see our discussion of nuclear structure. |
Products of Nuclear Reactions
|
The products of nuclear reactions normally consists of gamma rays,
some of the light particles (n, p, d, t, 3He,
α), and heavier "residual nucleus." Therefore, we
can define a nuclear reaction by specifying the projectile, target, and
products in forms like the following:
6Li(n,γ)7Li 235U(n,2n)234U The gammas aren't always given explicitly in these notations. In these cases, the residual nucleus is another stable isotope; however, sometimes it is unstable and decays with some "half life" into other products. For example, the (n,&gamma') reaction on 27Al produces 28Al, which decays to 28Si by emitting a negative beta particle with a half life of 2.25 minutes. Sometimes the break up of the residual is so fast that it doesn't make sense to show it explicitly. Therefore, we write
and omit the unstable 8Be from the notation. In writing out these reactions, it is important to preserve the charge (Z) and mass number (A). In the beryllium example the charge and mass before the reaction are 4 and 10. The two neutrons and two alpha particles remaining after the reaction also total up to Z and A values of 4 and 10. The various libraries of evaluated nuclear reaction data normally use some standard set of identifiers to define reactions. As an example, the ENDF system uses MT=102 for the (n,&gammma;) reaction and MT=16 for the (n,2n) reaction. |
Cross Sections: What the Heck is a Barn?
|
The probability that a nuclear reaction will take place is measured in
units of "barns," where 1 barn equals 10-24 cm2.
This is a unit of area. You can visualize a target material as an array
of little disks. Larger disks would be easy to hit (large cross section,
large reaction probability), and smaller disks would be hard to hit.
Folklore has it that this term was invented when there were still lots
of farm boys in physics; an atom with a large cross section would be
"as easy to hit as the side of a barn." Early compilations of nuclear
cross sections were called "Barn Books," and the National Nuclear Data
Center still uses a barn as an icon (
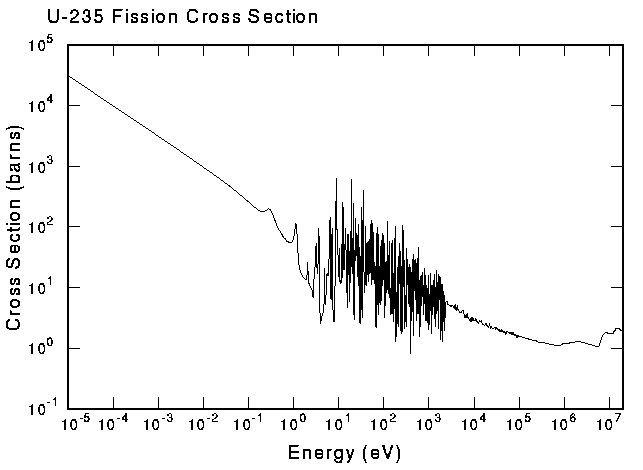
take a visit). As an example of a nuclear cross section, the following figure shows the fission cross section for U-235, the reaction that is responsible for generating a good fraction of the world's electricity. |

|
|
This is an example of an exothermic reaction--it releases energy.
Note that the cross section is very large at low energies. This is
where most of the reactions take place in a power reactor. The
complex structure at intermediate energies is from "resonances."
These are characteristic energies where the nucleus is easy to
excite, much the same as the characteristic vibration frequencies
of a guitar string. Endothermic reactions require energy to proceed; therefore, they can only be induced by incident particles that already have high energy. The minimum amount of energy required to induce such a reaction is called the "threshold" energy. Reaction thresholds are typically in the MeV range and higher. Below the threshold, the cross section disappears like the smile of the Chesire Cat. Clearly, the geometric analogy for reaction probabilities can't be followed too far! For an easy way to look up the reaction thresholds or the amount of energy used or released by nuclear reactions (the reaction Q value), try our qtool. |
Product Distributions in Energy and Angle
|
When a particle, such as a neutron or a proton, passes close to
a target nucleus, it can "scatter," changing its direction of
travel and its kinetic energy. The scattering can be "elastic"
or "inelastic". In elastic scattering, the target nucleus
remains in its ground state, but in inelastic scattering, it
can absorb energy from the incident particle (the absorbed
energy usually is re-emitted as gamma rays). Both of these
events are "two-body" collisions, much like billiard-ball
collisions, and the products fly off at different angles.
Mathematical analysis reveals that the energies of the scattered
particle and the recoil nucleus are completely determined by
the angle of emission of the scattered particle, and many of
these angular distributions have been measured or calculated
over the years. An example is shown in the following figure:
At the highest energies, the scattering is basically the diffraction of a wave around a hard sphere, and the results are similar to diffraction patterns for light. The forward peak (at Cosine=1.0) is like the bright central spot in a diffraction pattern, and the oscillations at larger angles (smaller cosines) are analogous to the fringes seen for light. It is also possible for the incident particle to merge right into the target nucleus, thus adding its mass and kinetic energy into an excited "compound nucleus." If enough time elapses before re-emission, the compound nucleus is said to come into equilibrium. You can visualize the incident particle bouncing around inside the nucleus until all its energy has been shared out with the other nucleons in the compound system. However, this compound system is "hot," almost like the tea kettle on your stove, and it can "evaporate" off particles just like the tea pot emits steam. The evaporated particles can be the same as the incident one, or different. For example, you might get an (n,2n) reaction, or an (n,p) reaction. If equilibrium has been reached, the particles in the compound nucleus will have bounced around enough to "forget" what direction the incident particle was going originally. The secondary particles will come off in all directions with uniform probability; this is call isotropic emission. The energy spectrum of the emitted particles will show a distribution similar in shape to emission of evaporated atoms from a liquid. Such shapes are characterized by the temperature of the medium, and they are called "Maxwellians" or "Maxwell-Boltzmann distributions" after the scientists who originally described them. At higher energies, particles do not always come into equilibrium before escaping from the nucleus. You could envision one just bouncing a few times and then coming out with much of its energy intact. This is called "preequilibrium emission." It is also possible for an incident particle of one type to transfer its energy to one of another type, and you could have preequilibrium proton emission from an (n,p) reaction. Preequilibrium particles "remember" more about their original direction, and they tend to come out in the forward direction. They also tend to come out with more energy than equilibrium emissions. An example of some of these effects is shown in the next figure:
The peaks at lower secondary energies are the "evaporation" protons, and the long shoulders at higher secondary energies result from preequilibrium emission of protons. This figure is an average over all emission directions, but a more careful look would show that the particles in the shoulders are emitted in the forward direction. This plot was made from the ENDF/B section section of our Data Area. |