|
The following article provides a brief introduction to the recent discovery of
deformed metastable superheavy nuclei and to theoretical calculations that had
predicted their stability and decay. It is available in printed form as T-2
Fact Sheet-1.
|
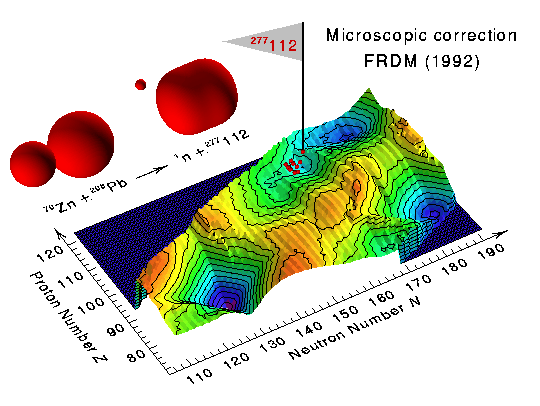
| The heaviest nucleus known to man, 277112, was discovered in February 1996 by scientists working at the Gesellschaft für Schwerionenforschung in Darmstadt, Germany. This nucleus, which consists of 112 protons and 165 neutrons, has a mass number of 277. It is the latest in a series of recently discovered nuclei lying on a rock of deformed metastable superheavy nuclei predicted to exist beyond the earlier chart of the nuclides. These newly discovered nuclei are shown as tiny red egg-shaped objects in the first figure, positioned according to the number of neutrons and the number of protons that they contain. Such superheavy nuclei exist only because of subtle quantum-mechanical effects leading to localized regions of nuclei with increased stability. |
|
|
|
The above figure shows 10 recently discovered superheavy nuclei, superimposed
on a theoretical calculation of the microscopic corrections to the ground-state
masses of nuclei extending from the vicinity of lead to heavy and superheavy
nuclei. Local minima in this quantity correspond to increased nuclear
stability arising from the closing of proton and neutron shells, such as occurs
near the doubly magic 208Pb nucleus in the lower left-hand corner.
The heaviest nucleus, whose location on the diagram is indicated by the flag,
was produced through a gentle reaction between spherical 70Zn and
208Pb nuclei in which a single neutron was emitted.
|
|
There occur in nature about 300 nuclei, representing isotopes of elements
containing from one to at most 94 protons. Some 2,200 additional nuclei have
been made artificially during the past 70 years. It becomes increasingly
difficult to make heavier nuclei because the disruptive electrostatic forces
between the positively charged protons grow faster than the cohesive nuclear
forces that hold the nucleons (protons and neutrons) together. The large
electrostatic forces cause heavy nuclei to decay rapidly by the emission of
alpha particles (helium nuclei) and by spontaneous fission.
Nuclei with increased stability beyond the earlier chart of the nuclides can exist because of the closing of proton and neutron shells. A nucleus with a completely filled shell of either protons or neutrons is said to be magic because it is relatively more stable than nuclei with either a larger or a smaller number of nucleons. Most magic nuclei are spherical in shape, but some nuclei can lower their energy somewhat, and hence increase their stability, by rearranging their protons and neutrons into deformed shells accommodating a different number of nucleons. The closing of these deformed shells leads to deformed magic numbers. By use of theories that reproduce the magic numbers and other properties of known nuclei, theorists have predicted that the next spherical proton magic number is 114 and that the next spherical neutron magic number is 184. In addition, they have predicted a deformed proton magic number at 110 and a deformed neutron magic number at 162. These predictions are made by solving the Schrödinger equation of quantum mechanics with an appropriate single-particle potential to describe the motion of the protons and neutrons. The results shown here are calculated from a realistic, diffuse-surface single-particle potential within the framework of the 1992 version of the finite-range droplet model (FRDM) of nuclear physics. |
|
|
|
The above figure shows the energy released in the alpha decay of the recently
discovered superheavy nucleus 272111 and its daughter nuclei. The
columns representing the energies are located on squares of the nuclear chart
corresponding to the alpha-emitting nuclei. The 1992 version of the
finite-range droplet model has accurately predicted the energy released in each
of these decays.
|
| Most of the metastable superheavy nuclei that have been discovered live for only about a thousandth of a second, after which they generally decay by emitting a series of alpha particles, as illustrated in the second figure for the superheavy nucleus 272111. However, the decay products of the most recently discovered nucleus 277112 show for the first time that nuclei at the center of the predicted rock of stability live longer than 10 seconds. By measuring the emission times and energies of these alpha particles, scientists can positively identify the initial superheavy nucleus that emitted the first alpha particle. The excellent agreement between these observations and theoretical predictions confirms the predictive power of current nuclear-structure models and represents a triumph for nuclear physics. |