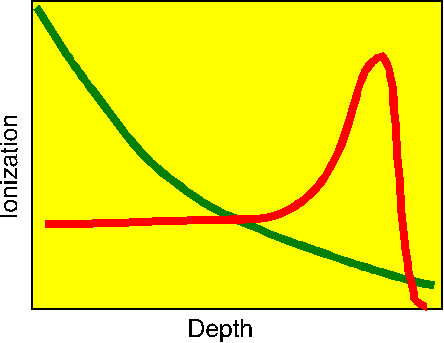
Note that most of the energy deposition comes near the end of the track. This is to be contrasted to the energy deposition curve for an x-ray (the green curve), where most of the energy is deposited early in the track (in the skin and muscle). The range of the proton can be adjusted by varying the energy of the beam so that most of them deposit most of their energy in the cancerous organ. Things can even be arranged so that the energy varies through an exposure, thus scanning the energy peak across the depth extent of the tumor.
This ability to localize the damage in the cancerous organ is clearly exciting. However, to make protons penetrating enough to reach deeply into the body, they have to be made quite energetic, with perhaps as much as 250 MeV of energy. Protons with these high energies can induce nuclear reactions in addition to the simple atomic slowing down discussed above. These nuclear reactions can deposit significant amounts of additional energy at the reaction site, and they can produce secondary particles, such as neutrons. The secondary neutrons, being neutral particles, can travel far from the reaction site and induce additional reactions. Thus, we have the possibility that the nuclear effects could enhance the treatment, or that they could cause significant collateral damage.
Our Group is working to provide new sets of nuclear data for neutrons and protons interacting with biological elements for energies up to 250 MeV. Clearly, the promise of proton therapy will require very sophisticated treatment planning methods. Livermore and Los Alamos both have programs to adapt some of the methods developed over the history of the weapons program to the needs of radiotherapy professionals. The general approach is to develop methods to take diagnostic scans from CAT or MRI machines and use them to construct a three-dimensional model of the target organ and surrounding structures. This model will then be used in a Monte Carlo transport calculation that follows the atomic slowing down and the nuclear reactions for all particles in full detail. Sophisticated graphical displays of the results would then become available to the radiologist in real time, thus allowing the exposure to be optimized with the patient in place. This work pushes the boundaries of nuclear data, radiation transport, computer technology, and interactive interface design. But it promises great payoffs in saved lives.
There are several sites on the Web with additional information on proton therapy and discussions of the work at particular medical centers.